Very nice to receive your inquiry.
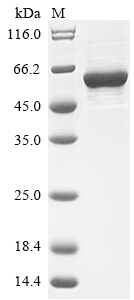
Recombinant Human Proto-oncogene tyrosine-protein kinase Src(SRC)
CSB-EP022650HU >> E.coli
Tag information:Tag type will be determined during the manufacturing process.
The expected tag is N-terminal 10xHis-tagged and C-terminal Myc-tagged.
Sequence:
GSNKSKPKDASQRRRSLEPAENVHGAGGGAFPASQTPSKPASADGHRGPSAAFAPAAAEPKLFGGFNSSDTVTSPQRAGPLAGGVTTFVALYDYESRTETDLSFKKGERLQIVNNTEGDWWLAHSLSTGQTGYIPSNYVAPSDSIQAEEWYFGKITRRESERLLLNAENPRGTFLVRESETTKGAYCLSVSDFDNAKGLNVKHYKIRKLDSGGFYITSRTQFNSLQQLVAYYSKHADGLCHRLTTVCPTSKPQTQGLAKDAWEIPRESLRLEVKLGQGCFGEVWMGTWNGTTRVAIKTLKPGTMSPEAFLQEAQVMKKLRHEKLVQLYAVVSEEPIYIVTEYMSKGSLLDFLKGETGKYLRLPQLVDMAAQIASGMAYVERMNYVHRDLRAANILVGENLVCKVADFGLARLIEDNEYTARQGAKFPIKWTAPEAALYGRFTIKSDVWSFPILLTELTTKGRVPYPGMVNREVLDQVERGYRMPCPPECPESLHDLMCQCWRKEPEERPTFEYLQAFLEDYFTSTEPQYQPGENL
Sorry, this is a semi-custom protein, we have never expressed any of these proteins before, neither have tried tag removal.
Please remark your requirement for tag removal if you need the untagged protein or tag removal when placing the order.
We can try enzyme digestion, but we can't guarantee 100% successfully. The overall success rate of enzyme digestion data analysis is 75%-86%.
Not all protein tags can be removed as some proteins will be very unstable after tag removal.
If we succeed in removing the tag, we will charge for extra cost.
If we fail in removing the tag, we won’t charge for any extra cost, and remark this information in datasheet as follows
“Note: The laboratory determined that the Tag on your protein could not be removed with standard laboratory procedures. Your protein is being supplied with the Tag intact.”
Generally, the delivery time will be extended for 3 days.
Sorry, as this is a semi-custom protein, which means we have never expressed any of these proteins before, so also haven't tested the activity, we can't 100% guarantee its activity.However, as what we provide is the full length protein, and it's purifie under very mild condition, we recommend you to purchase a small size of eukaryotic expressed protein to have a try. Generally, the more advanced expression system is the protein expressed with, the more likely to be active.
Reference: https://www.sciencedirect.com/science/article/pii/007668799100177X